Environment & Energy
Related: About this forumA Zirconium doped Copper-Zinc-Gallium Catalyst for Thermochemical CO2 Conversion to Methanol.
The paper I'll briefly discuss in this post is in the current issue as of this writing of Industrial Engineering and Chemistry Research is this one:
Zr-Doped CuZnGa Catalyst Increased Oxygen Vacancy to Promote CO2 Adsorption for Thermochemical Methanol Synthesis Weixin Liu, Tongyun Zhang, Zhuo Chen, Wentao Du, Yongbin Zhong, Jingwen Xu, Yuanfa Zhuang, Zhefeng Li, Hao Li, Shengxuan Luo, and Jun Cheng Industrial & Engineering Chemistry Research 2025 64 (22), 10656-10666.
I have always admired the 2011 paper by Nobel Laureate George Olah, published shortly before he died, advocating a closed carbon cycle via the hydrogenation of CO2 to methanol or (better) the wonder fuel DME, dimethyl ether: Anthropogenic Chemical Carbon Cycle for a Sustainable Future George A. Olah, G. K. Surya Prakash, and Alain Goeppert Journal of the American Chemical Society 2011 133 (33), 12881-12898
It has always struck me as what we should do, although what we should do is very unlikely, given the unfortunate propensity to do what we always have done, even as doing what we always do is destroying the planet.
What Olah proposed has nothing to do with primary energy, the production of which has one, and only one environmentally sustainable option, nuclear energy. However, this proposal is useful to make the intrinsic high energy density value of uranium, thorium, plutonium, neptunium and americium, portable, and, importantly, can do so in a setting of process intensification, in which the exergy value of heat can be raised to high efficiency, according to my own crude analysis, as well as some in the literature, to greater than 70%, with thermodynamically degraded electricity being a side product, as opposed to the main product, portable fluid fuels.
One of the elements in the catalyst is gallium, which is, somewhat obscurely, in the news, since the main reserves of this valuable element are in China, whence is where the authors of the paper are located. The scientific suicide of the United States owing to the orange ignoramus in the White House and his equally stupid supporters will further exacerbate Chinese accession to the world's superpower, which has long been underway. China has threatened, and actually may carry out, the suspension of gallium supplies to the increasingly uncivilized United States, as an economic sanction against fascism.
From the paper:
The use of an appropriate catalyst is a key point for hydrogenation of CO2 to methanol due to its high thermodynamic stability. Cu/ZnO-based catalysts have been extensively studied in the field of CO2 thermocatalytic methanol production due to their low cost and high methanol synthesis efficiency. (4) In these catalysts, metallic Cu is the active component, while ZnO, as a typical support, acts as a structural and electronic promoter. (5) The Cu/ZnO interface provides the key active sites for CO2 hydrogenation to methanol due to the strong metal–support interaction (SMSI) effect between Cu/ZnO. (6,7)
The single Cu/ZnO-based catalyst has low CO2 adsorption ability and poor methanol selectivity. (2,8,9) Moreover, the large size of Cu particles makes them prone to aggregation, which reduces the catalyst lifespan. Appropriate supports are beneficial for achieving SMSI between Cu/ZnO, which can modulate the oxidation state, size, and dispersion of active Cu species, thereby enhancing catalytic performance. (5,10) The addition of promoters such as Al2O3, (10,11) SiO2, (12) ZrO2, (13−15) and Ga2O3 (16−19) was reported to impore the stability and heat resistance of Cu/ZnO-based catalysts. In particular, the incorporation of Ga can significantly enhance the catalytic performance. Extensive research has been conducted on the mechanism of Ga doping in Cu-based catalysts. For instance, the formation of a thin layered double hydroxide (LDH) precursor structure in CuZnGa catalysts improved the dispersion of Cu particles on the catalyst surface. (17) The Cu-supported ZnGa catalyst prepared by microwave-assisted methods enhanced the SMSI between Cu/ZnO. (18) CuZnGa catalysts prepared under different pH conditions have different specific surface areas, which affects catalyst performance. (16) Ga also acts as an electronic promoter in Cu/ZnO catalysts, reducing activation energy and facilitating CO2 activation. (19) Additionally, Ga stabilizes the reaction intermediates and the intermediate states of Cu/ZrO2. (18)
ZrO2 has good thermal stability, which helps to improve the high temperature resistance of the catalyst and prevents the sintering and aggregation of metal particles. Moreover, ZrO2 strengthens redox properties and forms oxygen vacancies to help activate CO2 molecules. (20−23) So far, there has been no report on the CuZnGa catalyst doped with Zr. Therefore, this paper explores the modification of CuZnGa catalysts with Zr to address the issue of weak CO2 adsorption capacity...
I added the bold which makes, once again, George Olah's point. Hydrogen is a useful captive intermediate, which can be made by thermochemical cycles using nuclear heat (although it isn't except on a pilot scale in China - there is a difference between "could" and "is" ). The advertising, largely by the fossil fuel industry seeking to rebrand itself as "green," by claiming hydrogen is "green," here and elsewhere, of hydrogen as a consumer product, a stupid enterprise now more than a half a century old, is criminally insane. In China, as is the case everywhere else, well over 98% of the world's hydrogen is currently manufactured, with exergy destruction, from dangerous fossil fuels.
A graphic from the paper:
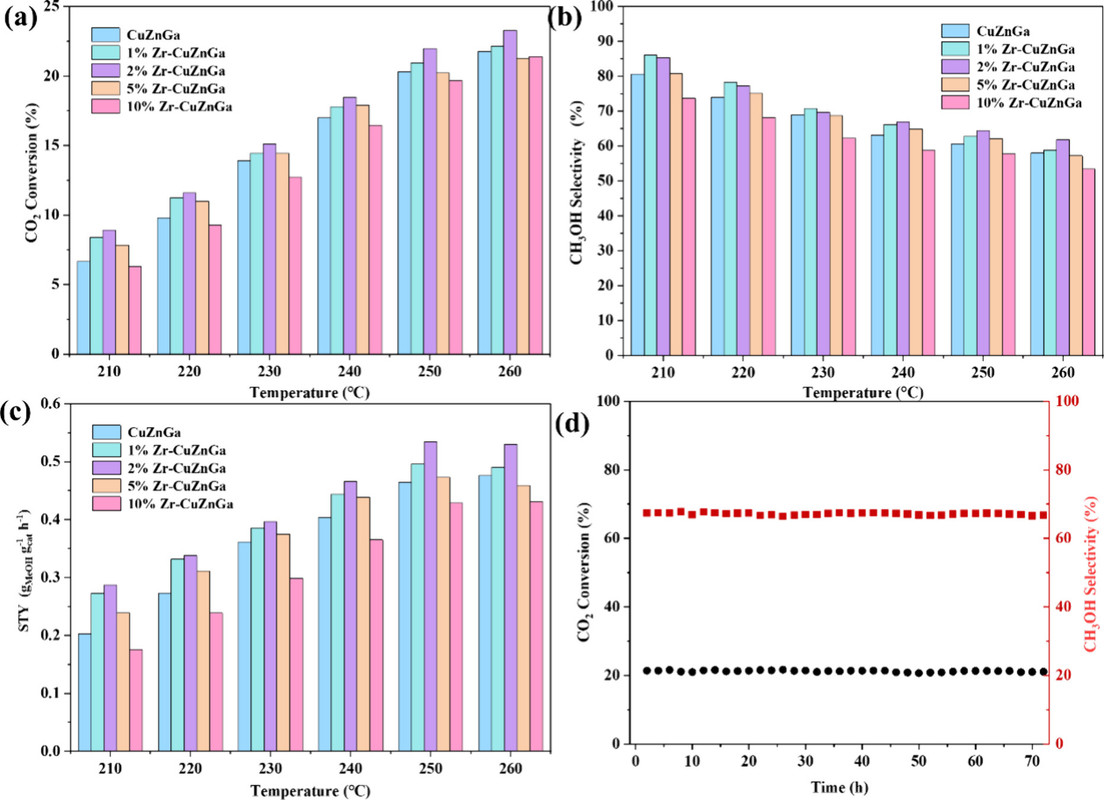
The caption:
The temperatures are accessible through heat networks driven by nuclear heat.
This is a lab scale process, and may or may not, the latter being more likely, advance to industrial scale.
It's nice however to see that the work is being conducted.
Have a pleasant Sunday.
Mopar151
(10,343 posts)Requires careful handling and storage! Will self-contaminate with humidity in any un-sealed container. Attacks many fuel system components that are compatible with gasoline or diesel. Has starting problems at low temperature (why E85 exists), some racers use a gasoline primer system. Lower energy density than gasoline - requires 2x minimum volume for equivalent output.
The pro's - makes mad power! Has a very high octane - compatible with diesel compression ratios, or high mechanical compression ratios in "spark ignition" engines - like 16:1in an "outlaw" sprint car engine! Higher thermal efficiency is a bonus. Similarly, the charge cooling effects of "alky" work wonders in turbo or supercharged applications. It's not just a "hot rod" thing, either - Maersk has ordered 6 container ships set up for methanol fuel!
NNadir
(36,983 posts)...natural gas in all applications.
It is an easily liquifiable gas with a critical temperature higher than the boiling point of water, 401K, is easily removed from water, is relatively nontoxic - slightly anesthetic - and has an atmospheric half-life of 5 days, and thus, unlike methane is not a climate forcing gas and lacks any corrosive properties, although for engines, some changes to seals would be necessary.
It is also an excellent refrigerant. It's thermodynamic properties on synthesis from hydrogen and carbon dioxide is slightly exothermic.
In short, it is cleaner than any other fuel, safer than another fuel, and more sustainable than any other fuel. It's essentially a "drop in" on existing infrastructure, unlike the hydrogen that the fossil fuel green washer comes here to sell.
I'm less concerned with its properties as a motor fuel than its sustainability. DME is a wonder fuel.