Environment & Energy
Related: About this forumDiscovering "Net Zero" Approaches to Matter and Energy Flows in Industrial Processes, a Methanol Case.
The paper I'll discuss in this post is the second paper in the current issue, as of this writing, of the Journal Industrial Engineering and Chemistry Research on the subject of methanol formed by the reduction of CO2 to give methanol.
This is it: Discovering Net-Zero Chemical Processes and Pathways by Developing Circular Reaction Networks and Their Hierarchical Screening Sunghoon Kim and Bhavik R. Bakshi Industrial & Engineering Chemistry Research 2025 64 (22), 10928-10949
I covered the first paper in this issue here: A Zirconium doped Copper-Zinc-Gallium Catalyst for Thermochemical CO2 Conversion to Methanol.
The authors are at Arizona State University which is, of course, subject to the willful destruction of American Science by the intellectual Lilliputians in the Republican cults. (One hopes they'll find some place to go to continue their work.)
I tend to focus on energy flows more than material flows, and thus often write of heat networks using nuclear heat as a primary energy source. This paper seeks to define matter flows in the chemical flow, in particular, carbon flows, with some focus C1/C2/H2 system which includes methanol, dimethyl ether (the wonder fuel), as well as higher alcohols, and in theory, all components current obtained by the isolation and transformation of matter found in dangerous fossil fuels.
It's a long paper, and I can only cover it briefly.
From the opening text:
Transitioning the chemical industry to a circular, net-zero enterprise necessitates a multifaceted approach, including novel technologies, innovative business strategies, and supportive policies. (7) Sen et al. proposed a comprehensive framework to support emerging technologies by integrating alternatives across all life cycle stages. This framework demonstrates significant potential to reduce global CO2 emissions and mitigate the industry’s environmental footprint by facilitating the transition to circular processes. (8−10) Thakker and Bakshi (11) explored the design of value chains for plastic and paper carrier bags, identifying pathways aligned with sustainability and circular economy principles. Employing a life cycle assessment approach, they evaluated the environmental impacts of various value chains and identified the most sustainable and circular options. Their findings underscored the critical role of circular economy systems in achieving net-zero emissions, emphasizing the need for integrating diverse technologies. However, while these research efforts provide valuable insights, the existing superstructure frameworks used to evaluate these alternatives are biased toward technologies that are either available or being developed. They are limited in their ability to discover innovative reaction pathways and emerging technologies that have not yet been widely studied or commercialized.
The premise underlying this work is that the vast knowledge base of chemistry could yield novel reaction networks to meet sustainability requirements, including net-zero emissions. Chemists have studied tens of thousands of reactions, but only a handful have been commercialized, selected primarily for maximizing profit. Recent years have witnessed significant advancements in integrating diverse chemical reactions into comprehensive frameworks. Given the new sustainability requirements, including net-zero emissions, it is time to revisit chemistry with a sustainable circularity lens to discover novel pathways for redesigning the chemical industry as a sustainable circular enterprise. Early efforts in this direction employ reaction network flux analysis to detect promising production routes and bottlenecks by integrating diverse alternative reaction data. (12) This method provides the foundation for identifying and optimizing new reaction pathways. Building upon this foundation, Ulonska et al. (13) introduced process network flux analysis, an extension of reaction network flux analysis, to evaluate from the process perspective, enabling the ranking of processing routes and identification of bottlenecks. Similarly, Thakker and Bakshi (14) extended this approach to larger scales with a framework integrating chemical reaction pathways, life-cycle value chains, and economic cash flows. Zhuang et al. (15) proposed a multiscale modeling approach linking chemical production processes with sustainability metrics, emphasizing metabolic, process, and ecosystem-level integration. Zhang et al. (16) developed a screening methodology to evaluate synthesis pathways for biomass-derived polymers, demonstrating the potential for sustainable polymer production. These foundational works underscore the untapped potential of underutilized reactions, particularly in achieving net-zero emissions. This exploration is further enhanced by integrating chemical databases, energy assessments, and advanced decision-making frameworks, which collectively facilitate the identification of optimal reaction systems. Batool et al., (17) although focused on drug discovery, highlight the potential of structure-based big data in optimizing chemical processes, a concept broadly applicable to chemical engineering. Innovations in automated reaction network optimization by Weber et al. (18) and autonomous reaction network exploration by Steiner and Reiher (19) demonstrate the utility of machine learning in discovering novel pathways. Mo et al. (20) leveraged chemical databases, patents, and a tree-LSTM neural network structure to identify promising retrosynthetic pathways, offering a data-driven approach for efficient prioritization. These advancements have culminated in tools such as ASKCOS and Aizynthfinder, (21) empowering chemists and engineers to navigate complex reaction networks with the potential to design sustainable solutions for net-zero emissions...
In other words, the authors propose a kind of data mining scheme, including some lab scale processes that may have been discovered but never industrialized, remarking that few of these ever reach the commercial scale. The public, and certainly here at DU as well, tends to look with some element of "magical thinking" that a particular problem is solved by appeal to a lab scale discovery, often breathlessly declaring a "breakthrough."
Personally, I have had the professional experience of making the first milligram of a particular compound, and, through a series of fortuitous coincidences of opportunities with business deals out of my control, shepherding it to commercial scale, a little over 10 tons. The ways that project could have, and might have failed were rather large; indeed, it actually happened that the process was one which, naïvely for everyone in our bench scale lab, would some day involve hundreds of metric tons, even thousands.
We were delusional.
Nevertheless, great inventions are often waylaid, forgotten, and in rare cases, rediscovered and advanced to prominence.
The authors here make a case for the novelty of their approach to data mining:
It is not for me to judge the accuracy of that claim; I'm not competent to do.
A table in the paper refers to a reaction network in the methanol system, which is not one I would have chosen, since it implicitly assumes access to dangerous natural gas, which I believe should be rapidly phased out, and includes both dry reforming, wet reforming, and seemingly, the rather dubious thermodynamic nightmare of electrolysis to make hydrogen, to which liars often appeal claiming it to be "green."
Nonetheless, here it is:
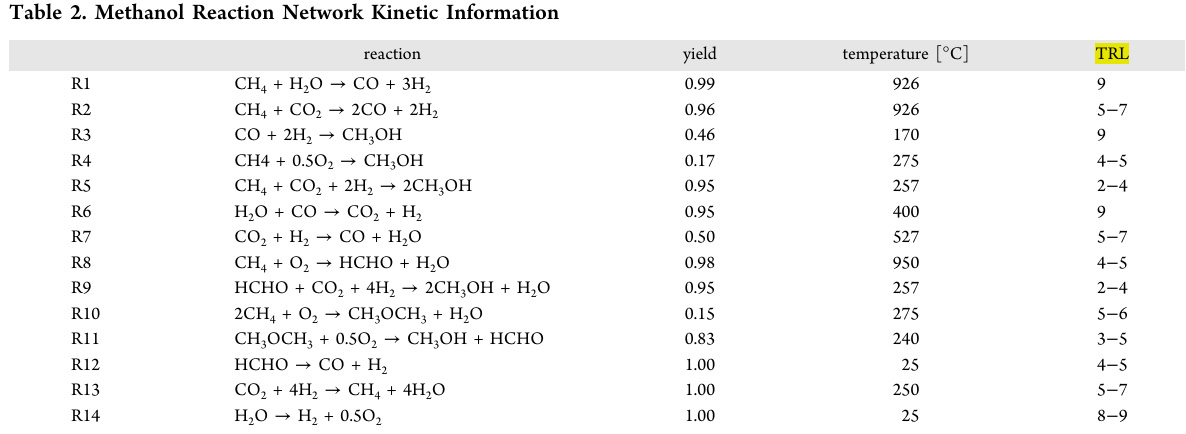
"TRL" refers to the authors' determination of "Technological Readiness Level," perhaps an important caveat depending on the criteria by which this parameter is determined. Steam reforming of dangerous fossil fuels to make hydrogen - which dominates world hydrogen production - is a well known industrial process, and as such, "R1," the steam reforming of dangerous natural gas, is given the highest "TRL," 9. So is electrolysis, "R14" although electrolysis, via thermodynamically degraded electricity is an environmental nightmare in practice, if not in widely reproduced rhetoric to appeal to public ignorance.
A useful feature of this chart, however, is a description of the temperature at which the reactions operate, representing a wide range, from 950°C in the partial combustion of methane to give formaldehyde and water, "R8" to room temperature 25°C, electrolysis.
While I personally find this list of reactions unsatisfying, the concept strikes me as useful.
A graphic from the paper represents some of the parameter space under which the system works:
The caption:
The "methanol" network:
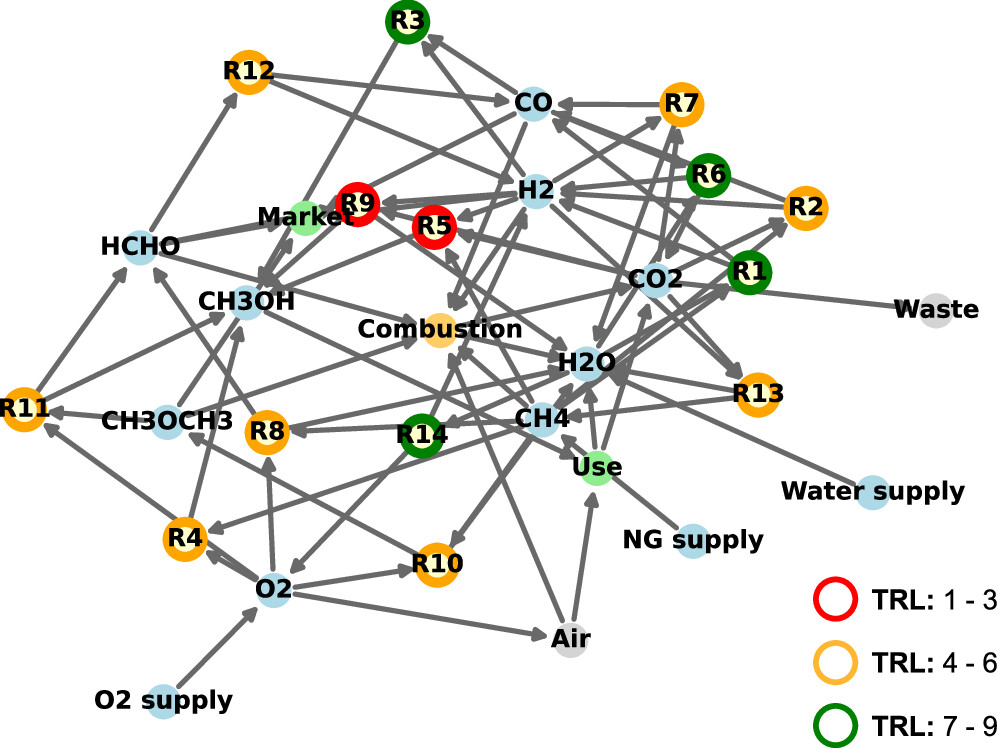
The caption:
Note that the input here is dangerous natural gas, meaning that this case is not sustainable in a clean and safe world, a clean and safe world decidedly not being the one in which we live. One could - perhaps with Chinese input, since they have assumed the technological preeminence that the United States abrogated in a last pulse of criminal stupidity represented by that orange creature in the White House - substitute carbon dioxide and water (with continuously recycled sulfuric acid and iodine in a closed cycle) as an input using nuclear heat, and again, with Chinese insight, understand the "TRL." In this case many of the reactions described in the table can be made to drop out (possibly to be substituted by others, for instance the dehydration of methanol to make DME) and displaced by the direct hydrogenation of CO2 to give methanol. Methanol, I note, can be a starting material for many products, including sustainable (multiuse) polymers via the well known and industrial "MTO" processes "Methanol to Olefins."
I've quibbled with much of what is in the paper, but I like the tone if not the inputs they use as an example. It strikes me as useful.
Have a nice day.