Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Environment & Energy
Related: About this forumA Rather Interesting Molten Salt.
I came across a paper about sodium batteries and found it interesting even though I'm not a battery fan kind of guy.
This is it: High-Voltage, Intermediate-Temperature, Fe- and Al-Mixed Metal Halide Molten Salt for Molten Sodium Battery Energy Storage Stephen J. Percival, Adam M. Maraschky, Melissa L. Meyerson, Matthew A. Stalcup, Amanda S. Peretti, Leo J. Small, and Erik D. Spoerke Industrial & Engineering Chemistry Research 2025 64 (47), 22714-22723.
From the text:
In recent years, there has been rapid progress in the transition to efficient and reliable energy storage systems that can handle evolving supply and demand needs on the grid. (1,2) As this transition gains momentum, safe, durable, grid-scale energy storage will be needed to bridge the mismatch between available supply and growing electrical demand. (3) These storage technologies must be able to store electrical energy during peak power generation periods and provide the power during peak loads to stabilize power generation and demand. (4) Historical energy storage with pumped storage hydropower (PSH) and rapidly emerging lithium-ion storage currently dominate the grid-scale energy storage landscape. (4−7) However, PSH can be practically challenging to implement in practice, and lithium-ion batteries face geopolitical supply chain issues, concerns over global lithium supply, and persistent risks of failure that can lead to fire and toxic gas release. (6,8−10)
Alternatives to lithium-based batteries, such as potassium- (11) and sodium-based batteries, offer comparable performance without the safety and supply chain risks of lithium-ion and are highly desirable. (1,12−21) Sodium-based batteries take advantage of the global abundance of sodium, (22−24) and circumvent many of the supply issues and economic constraints of lithium-based systems. (25) These sodium-based batteries have a long history of development, particularly in molten sodium systems, such as sodium–sulfur and sodium–metal chloride batteries. (12,13,15,16,26) Some sodium-based systems, such as those utilizing fully inorganic molten salt electrolytes, also potentially alleviate the dangers associated with lithium by tolerating overcharge/discharge events and exhibiting low-hazard failure modes. (6,26−29)
Fully inorganic molten salts are an attractive medium for electrochemical energy storage due to their high charge density, high electrical conductivity, and large potential stability windows. (30−34) Furthermore, these completely inorganic molten salts eliminate the flammable solvents found in other battery systems, potentially increasing safety. (16,35) Molten salts have been used to store energy in commercialized ZEBRA batteries employing an NaAlCl4 molten salt electrolyte with an Ni/NiCl2 metal cathode that is oxidized and reduced during cycling. (26,27,36−39) However, hurdles still exist with ZEBRA-type batteries, including the cost of the Ni, representing 63% of the total material cost of the battery (40) and how the insulating, low-solubility NiCl2 on the Ni particles leads to deactivation and capacity loss. (26,27,41,42) Many new alternative ZEBRA-like molten salt catholyte chemistries have been described, which aim to eliminate the previously mentioned ZEBRA battery limitations. (12−14,42,43) Some of these alternative molten salt chemistries operate at lower temperatures (44) and use less-expensive metal halides and metal cathodes, such as zinc, (40) iron, (14,41,45,46) copper, (28) and aluminum, (39,47) or aim to eliminate the ceramic membrane. (43) Operation at low-to-intermediate temperatures (less than 300 °C) can substantially lower operational costs and reduce component degradation, making an attractive target for new molten salt chemistries. (44,48,49) In particular, low-temperature molten salt batteries have demonstrated low-cost, high-energy density storage with high scalability. (16,35,50) These catholytes have been described in the literature and could increase battery capacity, lower operational temperature, and increase cycling current densities. (15,16,29,35) Promising catholytes utilizing Lewis acid-based chemistry have been developed in recent years. (15,35,51−53)
Here, we demonstrate an alternative, fully inorganic, high-voltage, molten salt battery catholyte based on earth-abundant iron and aluminum metal halides. The catholyte, composed of FeCl3/FeCl2, AlCl3, and NaCl salts, forms Lewis acid/base adducts, and certain compositions may show unknown eutectic behavior and melt at temperatures lower than those expected for the given salts. This catholyte is intended to utilize Fe3+/Fe2+ as the redox couple for charge and discharge, enabling the catholyte to remain completely molten, but a higher-than-expected cell voltage at a low depth of discharge (DoD) was found to have another higher-potential redox half-reaction, with chloride ions being utilized. Under both low and high DoD cycling conditions, the catholyte chemistry was evaluated to be stable and have high energy efficiencies.
Alternatives to lithium-based batteries, such as potassium- (11) and sodium-based batteries, offer comparable performance without the safety and supply chain risks of lithium-ion and are highly desirable. (1,12−21) Sodium-based batteries take advantage of the global abundance of sodium, (22−24) and circumvent many of the supply issues and economic constraints of lithium-based systems. (25) These sodium-based batteries have a long history of development, particularly in molten sodium systems, such as sodium–sulfur and sodium–metal chloride batteries. (12,13,15,16,26) Some sodium-based systems, such as those utilizing fully inorganic molten salt electrolytes, also potentially alleviate the dangers associated with lithium by tolerating overcharge/discharge events and exhibiting low-hazard failure modes. (6,26−29)
Fully inorganic molten salts are an attractive medium for electrochemical energy storage due to their high charge density, high electrical conductivity, and large potential stability windows. (30−34) Furthermore, these completely inorganic molten salts eliminate the flammable solvents found in other battery systems, potentially increasing safety. (16,35) Molten salts have been used to store energy in commercialized ZEBRA batteries employing an NaAlCl4 molten salt electrolyte with an Ni/NiCl2 metal cathode that is oxidized and reduced during cycling. (26,27,36−39) However, hurdles still exist with ZEBRA-type batteries, including the cost of the Ni, representing 63% of the total material cost of the battery (40) and how the insulating, low-solubility NiCl2 on the Ni particles leads to deactivation and capacity loss. (26,27,41,42) Many new alternative ZEBRA-like molten salt catholyte chemistries have been described, which aim to eliminate the previously mentioned ZEBRA battery limitations. (12−14,42,43) Some of these alternative molten salt chemistries operate at lower temperatures (44) and use less-expensive metal halides and metal cathodes, such as zinc, (40) iron, (14,41,45,46) copper, (28) and aluminum, (39,47) or aim to eliminate the ceramic membrane. (43) Operation at low-to-intermediate temperatures (less than 300 °C) can substantially lower operational costs and reduce component degradation, making an attractive target for new molten salt chemistries. (44,48,49) In particular, low-temperature molten salt batteries have demonstrated low-cost, high-energy density storage with high scalability. (16,35,50) These catholytes have been described in the literature and could increase battery capacity, lower operational temperature, and increase cycling current densities. (15,16,29,35) Promising catholytes utilizing Lewis acid-based chemistry have been developed in recent years. (15,35,51−53)
Here, we demonstrate an alternative, fully inorganic, high-voltage, molten salt battery catholyte based on earth-abundant iron and aluminum metal halides. The catholyte, composed of FeCl3/FeCl2, AlCl3, and NaCl salts, forms Lewis acid/base adducts, and certain compositions may show unknown eutectic behavior and melt at temperatures lower than those expected for the given salts. This catholyte is intended to utilize Fe3+/Fe2+ as the redox couple for charge and discharge, enabling the catholyte to remain completely molten, but a higher-than-expected cell voltage at a low depth of discharge (DoD) was found to have another higher-potential redox half-reaction, with chloride ions being utilized. Under both low and high DoD cycling conditions, the catholyte chemistry was evaluated to be stable and have high energy efficiencies.
This combination of salts, using some of the most "earth abundant" elements, aluminum, iron, sodium, and chlorine, melts apparently at around 160oC. I'm somewhat surprised I never heard of it. The cell was operated at a slightly higher temperature 180oC.
There have been lots of discussions of chloride based salts in nuclear applications. I generally am not fond of them because of the accumulation of the radioactive isotope 36Cl from neutron capture in one of chlorine's stable isotopes. 36Cl has a rather long half-life, which potentially would generate festivals of angst in antinukes; even though the collapse of the planetary atmosphere from fossil fuels produces no such angst among that benighted set.
Nevertheless, this battery can either be used for electrical energy storage, or alternatively, thermal energy storage, which is somewhat less thermodynamically suspect. I personally have no real problem with 36Cl, but there's no value in necessarily tweaking the beast of antinukism.
The authors claim, at least for early cycles at low current density, high electrochemical efficiency.
Some figures from the text:
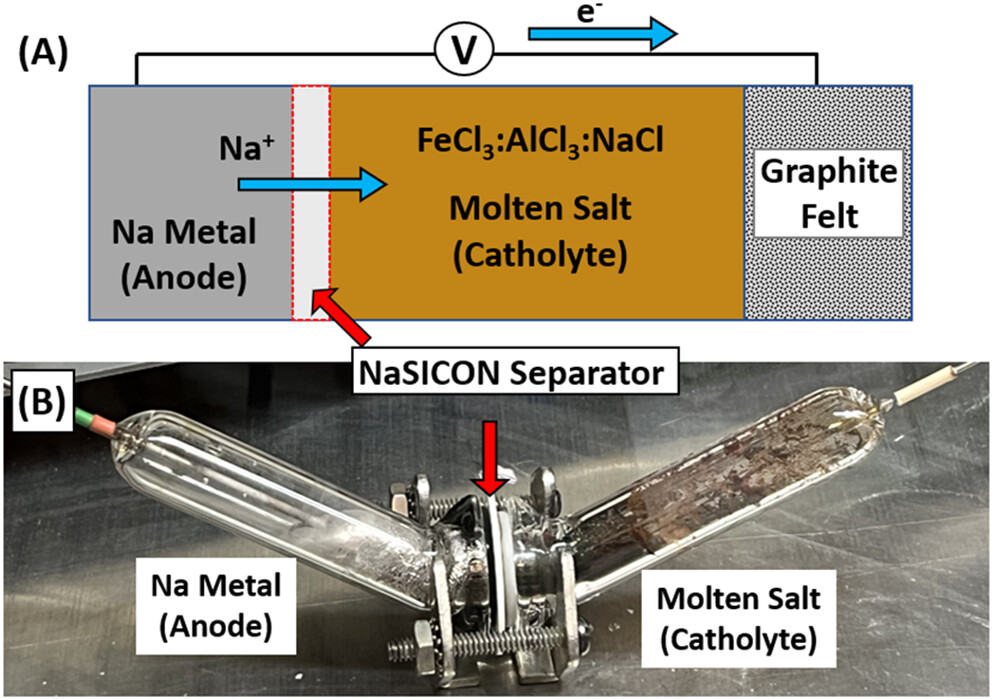
The caption:
Figure 1. (A) A simplified schematic of the battery with the molten Na anode and Fe-based molten salt catholyte separated by a NaSICON separator. (B) Photograph of the fully assembled battery test cell.
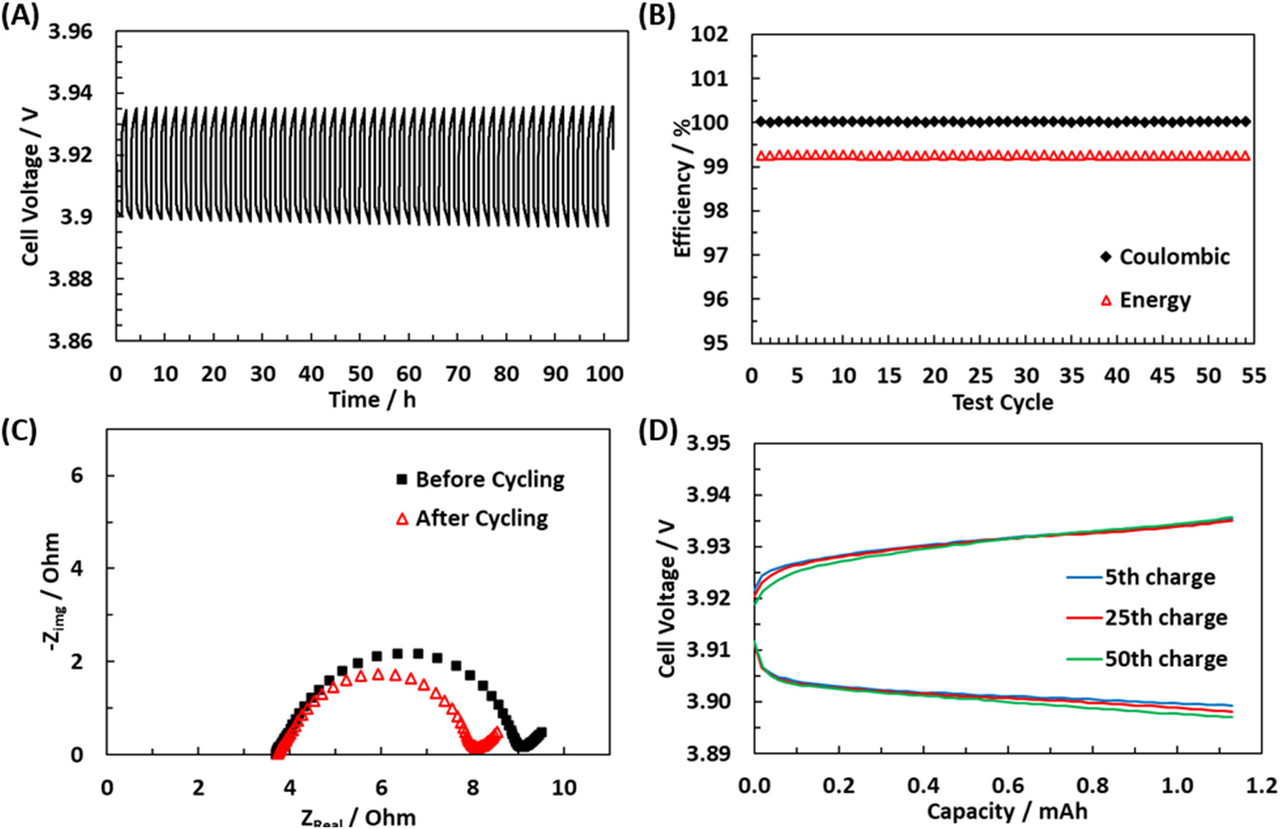
The caption:
Figure 4. (A) Battery cycling behavior of the cell with a 20:35:45 (FeCl3:AlCl3:NaCl) molten salt operating at 180 °C. Battery was cycled for greater than 50 cycles at 1 mA/cm2 with 1 h charge/discharge cycles. (B) Coulombic and energy efficiencies of the cycles in (A). (C) Impedance before and after cycling and (D) voltage–capacity curves for a few select cycles throughout the course of the testing.
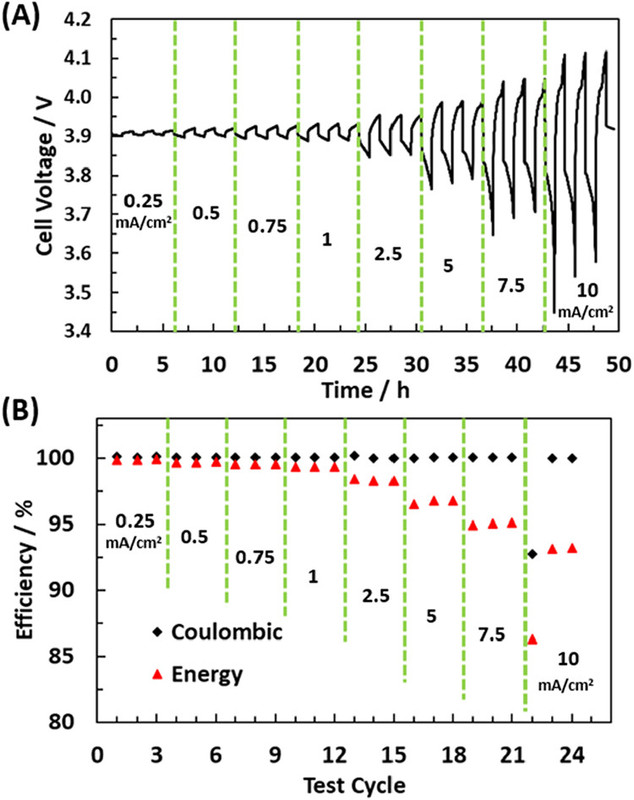
The caption:
Figure 5. (A) Voltage profile for a current density cycling rate test of the battery in Figure 4 (20:35:45─FeCl3:AlCl3:NaCl molten salt operating at 180 °C) with increasing current densities. (B) Resulting Coulombic and voltage efficiencies from the increased current density cycling test seen in (A).
Some remarks from the conclusion of the paper:
We have demonstrated a new and completely inorganic molten salt catholyte composition that utilizes earth-abundant Fe and Al metal halides, with Fe3+/Fe2+ being the targeted electrochemical redox pair. The tested composition, having a theoretical gravimetric capacity of 50.83 Ah/kg and a specific energy of 176.95 Wh/kg when paired with a Na anode, showed excellent energy efficiencies and stability when cycling. The initial investigation of the molten salt phase behavior revealed some compositions that were nearly fully molten at intermediate temperatures. The electrochemical behavior of the salt showed that the iron chloride species in the salt could be cycled but temperature could become important for increased performance. The battery, at an operating temperature of 180 °C and at low DoD, displayed cell potentials rivaling that of lithium-ion batteries while utilizing lower-cost materials. The new catholyte chemistry shows different cycling cell potentials based on the DoD of the battery and good cyclability, with high efficiencies demonstrated from both regions. The electrochemistry helped reveal the oxidation of Cl- was playing a role in the higher-than-expected operational voltage...
...The catholyte is expected to perform even better at the higher 210 °C temperature due to the increased solubility of FeCl2 in the melt. However, the higher-temperature cell design that eliminates the polymeric O-rings, which prevent higher-temperature testing, would require significant new materials development (e.g., glass seals) and lead to increased cell costs. As an alternative, new developments in molten salt composition could decrease the melting temperature of these salts and/or increase the solubility of the FeCl2 at lower temperatures, increasing the observed performance while allowing the continued use of this demonstrated low-temperature and lower-cost cell design. Further testing and optimization of the molten salt compositions will enable the control over the selectivity of the species in the melt and the desired operational cell potential.
...The catholyte is expected to perform even better at the higher 210 °C temperature due to the increased solubility of FeCl2 in the melt. However, the higher-temperature cell design that eliminates the polymeric O-rings, which prevent higher-temperature testing, would require significant new materials development (e.g., glass seals) and lead to increased cell costs. As an alternative, new developments in molten salt composition could decrease the melting temperature of these salts and/or increase the solubility of the FeCl2 at lower temperatures, increasing the observed performance while allowing the continued use of this demonstrated low-temperature and lower-cost cell design. Further testing and optimization of the molten salt compositions will enable the control over the selectivity of the species in the melt and the desired operational cell potential.
I doubt that the reported energy efficiency shown in the graphics accounts for heat loss from the molten salt.
An interesting paper, nonetheless I think.
I shared it with my son, who is working with earth abundant radiation resistant alloys designed for nuclear applications.
Have a pleasant work week.
2 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
A Rather Interesting Molten Salt. (Original Post)
NNadir
Sunday
OP
3catwoman3
(28,394 posts)1. Most of the time, I feel fairly intelligent.
And then, there are times like this.
NNadir
(37,050 posts)2. In my experience and opinion, the best way to BE intelligent is to spend time feeling unintelligent.
I have learned to spend my life trying to be the dumbest person in the room. Doing so makes it harder to do so.