Science
Related: About this forumInnovative Supramolecular Crystals Unlock High-Capacity Hydrogen Storage

Innovative Supramolecular Crystals Unlock High-Capacity Hydrogen Storage
Bioengineer.org | September 6, 2025
In the relentless pursuit of sustainable energy solutions, hydrogen has emerged as a linchpin in the transition away from fossil fuels. However, one of the most persistent challenges that has hampered the widespread adoption of hydrogen-based systems is the effective storage of hydrogen in a manner that balances capacity, safety, and practicality. Recent advances, spearheaded by innovative research into engineered supramolecular crystals, are poised to transform this landscape, offering a breakthrough that could accelerate the integration of hydrogen as a clean energy vector across multiple sectors.
Hydrogen storage, by its very nature, demands materials that can deliver both high volumetric and gravimetric efficiency. Traditional storage methods—whether compressed gas, liquefied hydrogen, or metal hydrides—have struggled to meet the dual criteria necessary for practical, scalable applications, especially in mobile and aerospace technologies. The recent work reviewed in a compelling perspective by Jiayi Zuo, Hao Wang, and Hongyi Gao delves into cutting-edge research conducted by Stoddart and colleagues, published in Nature Chemistry, highlighting how supramolecular crystals engineered at the molecular level offer a promising alternative.
The crux of this advancement lies in the supramolecular assembly of hydrogen-bonded organic frameworks (HOFs). Unlike conventional porous materials, these HOFs leverage the precise and directional multivalent hydrogen bonding interactions to self-assemble into highly ordered crystalline architectures. This rearrangement not only creates a stable yet reversible framework but also tunes the pore environments at the molecular scale, enabling optimized hydrogen uptake and release under dynamic conditions.
What’s particularly noteworthy is the dual achievement in volumetric and gravimetric capacities, quantified at 53.7 grams per liter and 9.3 weight percent, respectively. These figures are compelling benchmarks within the hydrogen storage community, establishing that engineered supramolecular crystals can circumvent traditional trade-offs that have long restricted material candidates. The dynamic thermo-pressure cycling tests further buttress these findings, demonstrating that these materials are not only effective under ideal static conditions but maintain performance integrity through real-world usage scenarios...more
https://bioengineer.org/innovative-supramolecular-crystals-unlock-high-capacity-hydrogen-storage/
NNadir
(36,905 posts)I suppose the point of the post was to make another scammy claim that the horrible physical properties of hydrogen that make it dangerous as a consumer product can be overcome.
However, this said, the chemistry found in the reference from the news item can be found here:
Zuo, J., Wang, H. & Gao, H. Engineered supramolecular crystals for high-capacity hydrogen storage. Front. Energy (2025) (ASAP, On Line July 10, 2025)
This is triptycene chemistry. The structure of the monomers is given in the paper as follows:
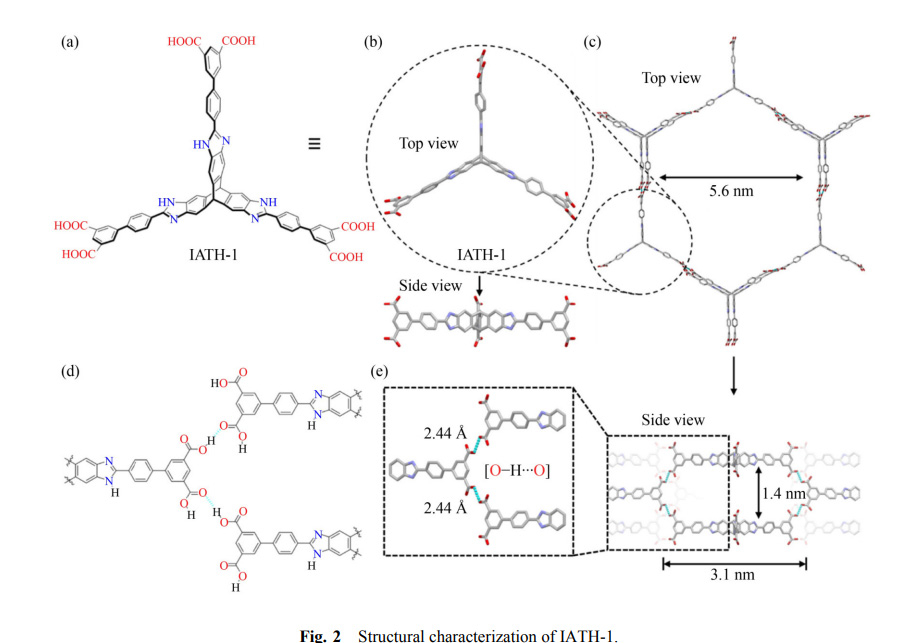
This isn't a simple triptycene, of course; the phenyl moieties are benzoimidazoles. Obviously they have been synthesized, but probably not at a meaningful scale subject to industrialization piloting.
Triptycenes are generally synthesized from 4+2 cycloadditions between anthracene - found in coal tar - and 2,3,4,5 cyclohexyldienes, somewhat problematic compounds prone to aromatization, but sometimes accessed through hydroquinone chemistry.
The fact that these molecules have as their basic source, dangerous fossil fuels is consistent of course, with the fact that marketing here of hydrogen is nothing more than an effort to greenwash fossil fuels.
No one is going to make 100 million tons of these triptycenes. The chemistry, on an industrial scale, would be awful.